A CHANCE ENCOUNTER next to the Complex Systems Research Center mailboxes led to a pre-eureka moment for Ruth Varner.
Early last year her CSRC colleague, tropical ecologist and sound technologist/musician Michael Palace, was opening a package he had just received when Varner passed by. “Guess what I got?” Palace asked Varner, whom he knew was interested in field recordings he’d done during research projects in tropical forests. “A hydrophone,” he answered.
Palace had purchased the underwater microphone to record waves and dolphins in the Caribbean, but in Varner’s mind a different thought quickly surfaced. “Do you think we could hear bubbles with that, bubbles of methane from peatlands?” “Let’s find out,” replied Palace.
With no peat readily available, the two grabbed a couple of paper coffee cups, a few little stirrer-straws with which to blow bubbles, and headed to Palace’s office. They plugged the hydrophone into the requisite electronic gadgetry he had on hand, plunged it into a coffee cup filled with water, and commenced to blow bubbles. Eureka!
Some months and a lot of front-end work later, Varner and Palace received a pilot grant of $50,000 from the U.S. Geological Survey to further develop and field test instrumentation that could listen for methane and, potentially, help provide insight into one of the biggest unknowns in the climate change puzzle: the global methane budget. Specifically, Varner and Palace’s unique effort will attempt to nail down the methane that is emitted to the atmosphere through the process of “ebullition” or bubbling.
Wetlands are the planet’s largest natural source of methane and ebullition is perhaps the dominant pathway for its release. However, measuring the bubbling process is so challenging that its estimated contribution to the global budget runs the gamut from zero to 60 percent as gauged by a handful of field studies.
“It’s a really hard number to get at,” Varner says, “there have been a number of estimates but the error bars are really large because ebullition happens both spatially and temporarily in a very heterogeneous way.”
It is difficult, in other words, to know where and when a methane bubble is going to burst. And, one wonders, even if it’s possible to take measure of a single bubble by sound, how do you to gauge its frequency, volume, and density to derive how much methane is trapped inside before it adds its two cents worth to the global picture?
 |
|
| Martin Wik, a graduate student at Stockholm University, installs bubble sensors in Mellan Harrsjön, a lake adjacent to Stordalen mire. Photo by Ruth Varner, UNH-EOS. |
That is, in fact, precisely what Varner, Palace, and master’s student Jillian Lennartz have figured out how to do, and they are currently in the process of bringing the technique to fruition. Field tests were conducted last fall and an array of instruments will be deployed this summer in wetlands locally, in Alaska, and in Sweden (see related story “Extending the Reach of Research”).
Lennartz is currently conducting rigorous calibration testing in the lab so that the scientists can be assured next season’s field data is spot-on, and Palace is working on the sound-side of things writing computer code that will aid in the accurate and efficient interpretation of a methane bubble’s sound signature vis-à-vis the minute details of the gas itself.
One Size Does Not Fit All
Using what’s known as an autochamber technique, Varner has been tracking methane emissions at a 9,000-year-old wetland area known as Sallie’s Fen in Barrington, N.H. since 2009 and UNH scientists have a record of methane emissions from the fen going back 22 years. (A fen is a peat-forming, groundwater-fed wetland thick in vegetation. Autochambers open and close automatically to take precise measurements of the buildup of gaseous emissions over a specified period of time.)
Although she and graduate student Jordan Goodrich have been using the autochambers to measure total methane emissions via diffusion from soil, water, and plants, they began to suspect that ebullition was occurring because data repeatedly showed abrupt spikes in methane levels.
Says Varner, “We’d see a rapid increase in methane concentration in a short period of time, which indicated a large amount was being released during an ‘event.’ So we realized ebullition was not as episodic as we originally thought, but actually happens pretty frequently and at a regular rate.”
Varner also realized they couldn’t deploy an army of the large, expensive autochambers to capture tiny bubbles, so the arrival of Palace’s hydrophone in Morse Hall was fortuitous indeed.
Another bit of serendipity was Lennartz showing up at Varner’s office door in search of a master’s project. With a background in chemistry and computers, Lennartz was a good match for Varner’s nascent ebullition research/instrument development, and the fact that she had a mechanical engineering background “by proxy,” as she puts it, made her a shoe-in for the project.
“My father has two degrees in engineering and a machine shop at home. I grew up with spare robotics parts scattered around the house. He was always trying to make a robot that would serve drinks or do some other mundane task,” she says.
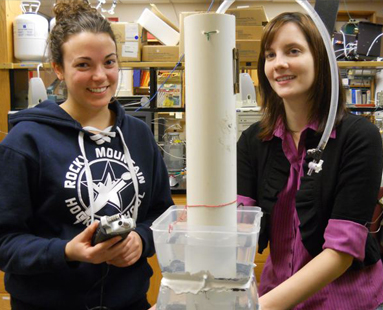 |
|
| Undergraduate Jacki Amante (left) and Jillian Lennartz conduct calibration tests on the hydrophone-based ebullition instrument invented at UNH.Photo by David Sims, UNH-EOS |
For her master’s thesis work, Lennartz was tasked to build and test out a variety of designs for hearing and analyzing bubbles of methane. The plan was to find an inexpensive and practical way of conducting the science. With input from Varner and Palace she built one gadget using a funnel stuffed with vibration-sensitive material. Another attempt was made using a stethoscope that had been gutted and replaced with a tiny, inexpensive microphone that Palace had made previously to record the hundreds of little “footsteps” of centipedes in Amazonian rainforests. (The miniscule and affordable technology is the same often used by performance artists to incorporate sound with body movements.)
The first recorder didn’t produce a strong enough bubble signal, while the latter tuned in a riot of extraneous noise. So in the end the group came full circle, back to the original hydrophone-based instrument that Palace and Varner had tested in his office half a year earlier.
After going through several iterations Lennartz ended up with an instrument comprised of a side-hinged PVC pipe that, when opened, exposes two, small offset funnels – one containing the hydrophone itself, and one off to the other side and higher up that captures the bubble after it hits the hydrophone. Completing its journey, the bubble rises up a tube into a “gas trap.”
Explains Lennartz, “Periodically we’ll collect the gas in the trap, put it in a vial, and look at the concentration of methane and its the isotopic signature so we can tell something about how the methane is being produced. The deeper, older methane will be heavier, the stuff closer to the surface, lighter. We’ll do the analysis in the isotopic lab here at UNH.”
 |
|
| Jillian Lennartz installs a recording device for an ebullition sensor at Sallie's Fen in Barrington, N.H.Photo by Olivia Varner. |
Lennartz notes that the method the team came up with to sample methane ebullition allows them to accurately measure the when, where, and how of the process.
“The great thing is that when the bubble is recorded we’ll know if it occurred at night or during the day, and under what meteorological conditions. It’s a relatively complete understanding of the entire process – where it’s produced, how it’s released, and what causes it to be released.”
With such a fundamental data set in hand the trick will then be to figure out what it all might mean in terms of filling in the blanks with respect to global methane sources, which is the ultimate goal of the project and will be the particular focus of future funding that Varner and Palace are pursuing.
Says Varner, “That’s actually something we’ll be addressing, How do you scale these data up and generalize what this instrument can do in an array in one specific location to derive an estimate for methane ebullition for the entire globe?”
It will be a key question to answer; methane’s role in the climate change puzzle could increase significantly in years to come as areas of permafrost thaw and peatlands degrade to potentially release much more of the potent climate-forcing gas.

